 |
Centre for Australian National Biodiversity
Research
|

Nathan Potter
Email: nathpotter@hotmail.com
Home: (02) 6161 9267
Mobile: 0404 30 7733
Supervisors: Augusto Becerra & Curt Brubaker
Fusarium wilt is an increasingly destructive disease for cotton production in Australia and abroad. It is caused by the fungal pathogen Fusarium oxysporum f. sp. vasinfectum (Fov), and developing disease resistance in commercial cultivars is imperative for the amelioration of the industry. This trial aims to develop and evaluate an inoculation method suitable for the purposes of genetic studies that investigate Fov resistance.
Australian native cotton plants and commercial cultivars were inoculated by growing them in seedling trays of potting mix infested with Fov-colonised wheat seed. The Fov–wheat inoculum was mixed with potting mix in different proportions to make up six treatments that ranged in colony forming unit (CFU) densities from Fov free (as a control), then 5 x 104 to 5 x 106 CFU/g.
The treatments of CFU densities closer to that of field levels, treatment 2 (5 x 104 CFU/g) and treatment 3 (1 x 105 CFU/g), produced consistent disease symptoms that strongly correlated with cultivar resistance observed in the field. This inoculation method allows efficient and accurate screening of Fov resistance in the glasshouse, and has potential to advance genetic research in the pursuit of developing disease resistance in commercial cultivars.
Fusarium wilt is a cotton disease that causes devastating losses in most countries where the crop is grown. Australia was thought to be free of Fusarium wilt until March 1993 when the disease was confirmed in the Darling Downs of Queensland. Since then, it has rapidly spread to a large proportion of the cotton growing areas in the country. The wilting disease is caused by Fusarium oxysporum f. sp. vasinfectum (Fov), which is a soil and water borne fungal pathogen. The symptoms it produces in cotton plants encompass leaf necrosis, vascular discolouration and stunted growth, and in extreme cases stem and leaf death occur. (Kochman – Living with Fusarium Wilt)
The Fov pathogens that are responsible for Fusarium wilt in Australia have been found to differ from those elsewhere. Abroad, Fov is favoured by acidic sandy soils and the presence of various nematodes, whereas Australian Fov is favoured by alkaline dark grey heavy clay soils and does not require nematodes for severe disease. (Bell, Wheeler, Lui, et. al., 2003) The Australian pathogen is divided into two vegetative compatible groups (VCGs 01111 and 01112), which are vegetatively incompatible with each other and with Fov isolates from any anywhere else. They are genetically unique and have been found to be of indigenous origin. (Wang, Brubaker and Burdon 2004)
Since Fusarium wilt is increasingly destructive in cotton production, ways of controlling the disease need to be developed. Evidence suggests that growing resistant cotton varieties may reduce the rate at which the disease builds-up and spreads in the fields, and is a way of controlling Fusarium wilt. (Wang, Kochman et al., 1997) Although the new commercial cultivars developed over the past 10 years have increased their resistance to Fov, there is still much need for improvement. Progress has been hindered by the limited sources of Fov resistance available within the elite Australian germplasm pool and the lack of a suitable glasshouse screening technique. As an alternative source of resistance, the native Australian Gossypium species are being investigated, as it has most likely co-evolved with the indigenous Fov pathogens. (McFadden, Beasley, and Brubaker 2004)
Various inoculation methods for glasshouse screening of resistance have been developed, but all have their shortcomings and the lack of a suitable glasshouse screening technique remains.. The success of screening strains of cotton resistant to Fusarium is dependent on the development a rapid technique that eliminates escapes and insures an accurate expression of resistance. It must give reproducible wilt reactions that correspond with known resistance among cotton varieties and correlate with field observations under natural conditions. (Bugdee and Sappenfield 1967)
In this study, we sought to develop a technique that will accurately screen large numbers of individual native and cultivated cotton plants, and be temporally and spatially efficient. To do this, a soil-amended colonised substrate method for screening Fov resistance in cotton was developed and evaluated. The ultimate objective is to have a standard glasshouse inoculation technique ideal for genetic studies that aim for an introgression of Fov resistance from the Australian native cottons.
Three kilogram lots of wheat seed were placed in flasks and soaked for 18 hours then they were drained and autoclaved at 121° C for 22 min in a slow exhaust cycle. The wheat seed was then incubated for two days to verify that the autoclaving was successful. Each flask was then inoculated using 15 ml of a conidial suspension (107 conidia/ml) of the Australian Fov isolate VCG 01111 (Acc. No. 24500). The inoculated wheat seeds were incubated for a period of two weeks to allow sufficient fungal growth. The inoculated wheat seeds were then dried, blended, and then stored in paper bags.
The Fov-wheat inoculum was thoroughly mixed through a potting mix (60% compost, 40% pertile) in different proportions and placed in seedling trays (57 x 28 cm) to make up 6 treatments: treatment 1 was control and no inoculum was added; treatment 2 had an initial inoculum load of 5 x 104 conidia/g; treatment 3 had 1 x 105 conidia/g; treatment 4 had 5 x 105 conidia/g; treatment 5 had 1 x 106 conidia/g; and treatment 6 had 5 x 106 conidia/g. A total of 9 kg of potting mix or inoculum amended potting mix was placed in each seedling tray. The seedling trays were arranged in the glasshouse in randomised block design with five blocks containing a set each of the six treatments.
Samples of infested potting mix, and potting mix from control trays, were first assayed for the pathogen 2 days after amendment and then assayed again, exactly six weeks after the first samples were taken, to measure the Fov dynamics. One gram samples of each potting mix treatment were serially diluted into sterile water, and then 1 ml aliquots of each dilution were plated in potato dextrose agar (PDA). Dilutions from the first assay were plated in PDA, but the dilutions from the second assay were plated in PDA with added streptomycin to eliminate background bacteria. Agar plates were incubated at 25ºC in the dark for 3 to 4 days. The number of Fov colonies on each plate was counted, and the inoculum density was expressed as colony-forming units (CFU)/g of dry potting mix. Agar plates produced from control trays were used to survey the microflora in the potting mix.
Seeds of the commercial Gossypium hirsutum cultivars, Siokra 1-4, Sicot 189 and Sicot F1 were germinated in the potting mix treatments. Siokra 1-4 is known as Fusarium wilt susceptible, while Sicot189 and Sicot F1 have been designated as Australian Industry standards for Fov resistance. Seeds of the native Australian G. sturtianum plants, Goss-5050 and Goss-5250, were nicked and pre-germinated on wet filter paper in the dark over 48 hours before being planted at the same time the cultivar seeds were sown. In previous studies, Goss-5050 has been found to be Fov resistant, and Goss-5250 has been found very susceptible. (McFadden, Beasley and Brubaker 2004)
Lines, consisting of 14 plants, of each cotton variety were randomly positioned on each seedling tray. The five blocks in the glasshouse contained a total of 2100 individual, i.e. 420 individuals per genotype.
Germination was monitored daily from time of sowing. After the plants were established (showing first true leaves), heights were measured weekly. At week eight, after sowing, the plants were harvested and rated for disease symptoms according to their vascular discolouration based on the scale: 0 = no vascular discoloration; 1 = discoloration restricted to base of stem only; 2 = discoloration of the "internode 0" (hypocotyl) region of the stem below the cotyledons; 3 = discoloration of stem above the cotyledons; 4 = complete vascular discoloration of stem; and 5 = plant dead. (McFadden, Beasley and Brubaker 2004)
Compared to initial inoculum loads, the first potting mix samples revealed a 2-fold CFU increase in treatments 2 and 3 showed, a 4-fold CFU increase in treatments 4 and 5, and nearly a 6-fold CFU increase in treatment 6. (Table 1) During the time between the first and second potting mix samples were assayed for the pathogen, the CFUs in treatments 2 and 3 decreased by nearly 10-fold, those in treatment 4 decreased 30-fold, treatment 5 showed a decrease of nearly 100-fold and treatment 6 showed a 300-fold decrease. (Table 2) Final Fov densities across all treatments declined towards stabilising at similar levels. (Fig. 1)
The microflora survey of the potting mix in treatment 1 (control) showed evidence of Aspergillus, Penicillium, Gliocladium and other Fusarium species. The second sample set taken from the control trays revealed the potting mix to be Fov contaminated, with a density of 1.7 x 103 CFU/g.
|
Table 1: First CFU survey |
Table 2: Second CFU survey |
|||
|
Treatment |
Increase (in multiples) of Fov density from initial inoculum load |
Treatment |
Decrease (in multiples) of Fov density between 1st and 2nd assays |
|
|
2 |
2.2 |
2 |
6.9 |
|
|
3 |
2.4 |
3 |
8.1 |
|
|
4 |
4.0 |
4 |
32.3 |
|
|
5 |
4.4 |
5 |
96.5 |
|
|
6 |
5.9 |
6 |
309.9 |
|
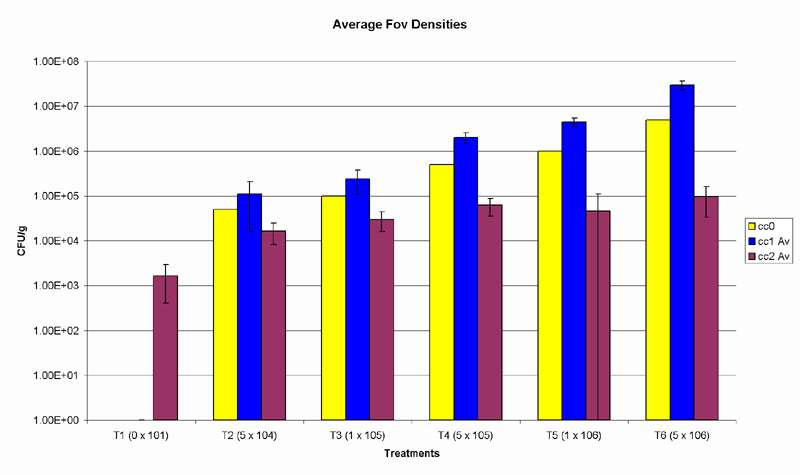
Fig. 1. Yellow bars represent the initial inoculum load of each treatment, blue bars show the results of the first soil survey, and the purple bars represent the final CFU densities. Y-axis is set to logarithmic scale. Error bars represent standard error of means.
The wild cotton varieties, Goss-5050 and Goss-5250, were germinated before being planted into the soil of all treatments. Therefore, no data was collected on their germination.
The germination of the susceptible cultivar, Siokra 1-4, significantly varied from the two more resistant cultivars Sicot 189 and Sicot F1, and was lower in most treatments; the germination between the two resistant cultivars, however, was not significantly different. Germination of all cultivars was highest in treatment1 (control), germination in treatment 2 fell by 10%, but was not significantly different. In treatment 3, germination was 20% lower than in the control and was significantly affected by the Fov density present in the soil. (Table 2) The germination in treatments 4, 5 and 6 was unreliable. (Fig 2)
Table 2: Multiple Comparisons ANOVA of Germination for Treatments 1, 2 & 3
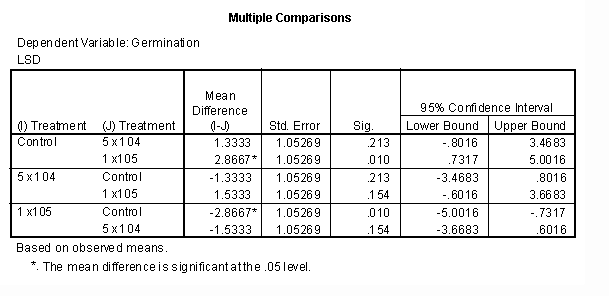
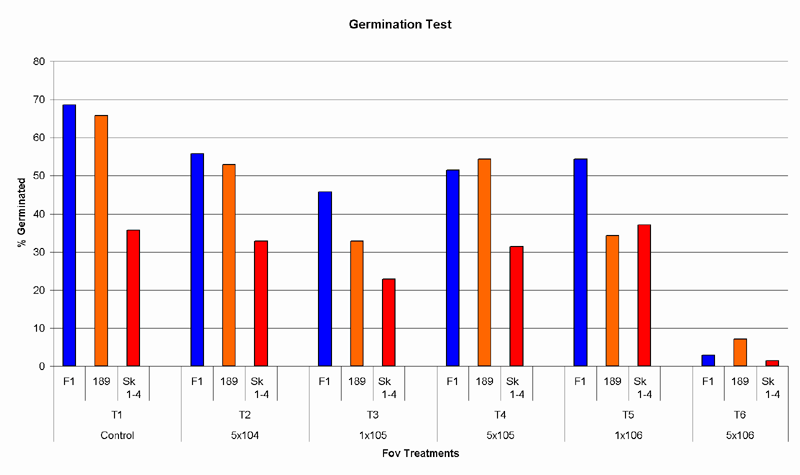
Fig. 2. Effect of Fov densities on germination. Germination is shown as a percentage against the number of seeds planted.
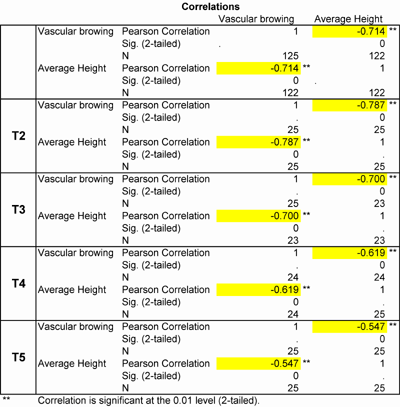
There was significant difference between the vascular discolouration observed in the control and in all other treatments. The vascular browning scores between the susceptible plants, Siokra 1-4 and Goss-5250, were not significantly different. Both of them showed significantly higher scores than the resistant plants, Sicot 189, Sicot F1 and Goss-5050. Sicot F1 showed significantly less vascular discolouration than Sicot 189. The vascular browning scores of Goss-5050 were slightly higher than Sicot F1 and slightly lower than Sicot 189, but these differences were not statistically significant. (Fig.3)
Treatments 2 and 3 were observed to strongly correlate with expected cultivar disease responses. (Table 3) In treatments 4 and 5, the decreasing disease incidence of the susceptible industry standard, Siokra 1-4, showed these treatments to be unreliable. The high Fov levels in treatment 6 prevented any substantial plant growth, and for this reason vascular browning scores were not measured for this treatment. Vascular discolouration was observed in the slightly contaminated controls, but no severe symptoms were apparent.
Table 3: Correlation between expected and observed vascular discolouration in Treatments 2 & 3.
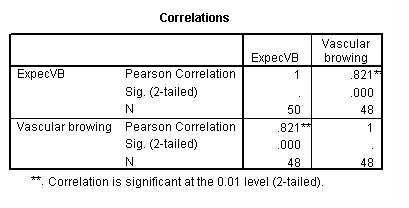
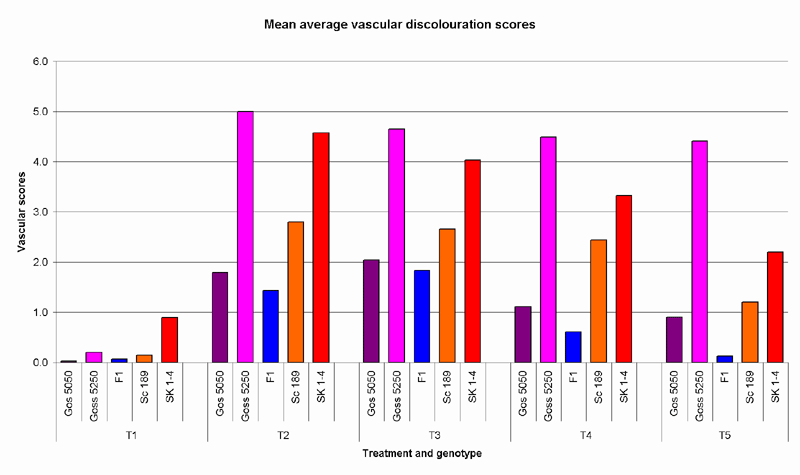
Fig. 3. Mean average vascular discolouration observed in cotton varieties across treatments 1 to 5.
A significant difference in growth rates was observed between the control and all other treatments. No significant difference of growth rates was observed between treatments 2 and 3, or between treatments 4 and 5. Growth rates were significantly different between all cotton varieties, except between Siokra 1-4 and Sicot 189, and Sicot 189 and Sicot F1. (Fig.4)
Growth rate performances negatively correlated with vascular browning scores; meaning that the higher the vascular browning score a plant had, the lower its growth rate was. Treatments 2 and 3 exhibited higher vascular browning – growth rate correlations than treatments 4 and 5. (Table 4) This correlation was not investigated in treatment 6, as plant growth was only observed for a short period on one of the five benches and data could not be sufficiently and accurately collected.
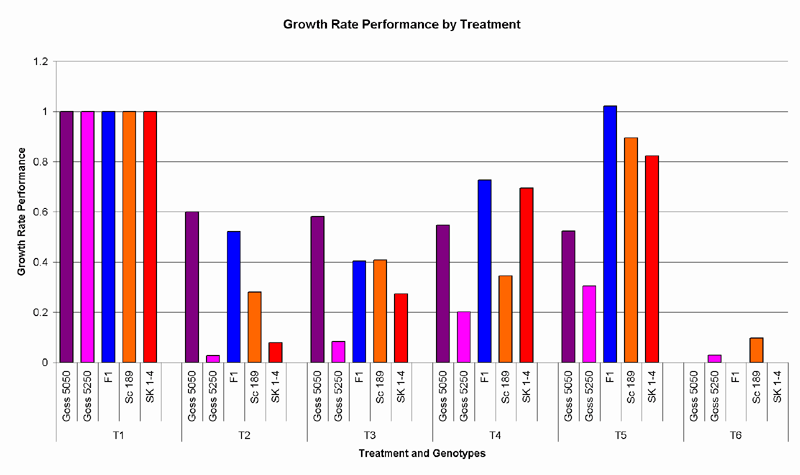
Fig. 4. Growth rate performances were calculated by taking the growth rates of each genotype in the Fov infected treatments as a percentage of those in the control.
Table 4: Vascular browning – growth rate correlation in treatments 2 to 5
The preliminary aim of this study was to evaluate the soil amended colonised wheat inoculation method by comparing it to the root dipping method. However, to increase the analytical power of this experiment, the soil amended colonised substrate inoculation method was studied in isolation.
The first CFU survey of the treatments revealed a Fov density increase proportional to the amount of wheat inoculum present in the treated potting mixes. This increase may be due to the high volume of organic matter (flour) in the inoculum. As a way of controlling this increase and having a more reliable pathogen density in each treatment, millet seed may be a better alternative to the wheat seed as an inoculum as it contains it has a lower volume of organic matter. The second CFU survey showed a decrease in Fov density that was proportional to the CFU levels initially found, i.e. CFU decreases were much more substantial in the higher concentrated treatments. The final Fov densities across all treatments appeared to decline towards similar levels. This result suggests that energy resources limit the sustainability of high pathogen levels in soils.
The potting mix in the control trays were shown to be contaminated in the second CFU survey. It has been found that F. oxysporum can be airborne in the glasshouse and is capable of reinfesting steamed soil (Elmer 2002), and Gracia- Garaz and Fravel report that spores are transported by running water. (Gracia- Garaz and Fravel 1998) The observed contamination of the controls may, therefore, be accounted for by airborne microconidia present in the glasshouse and splashing of water from one tray to the next during watering, which is most likely to be the cause of greatest significance. Placing the control trays on a separate bench in the glasshouse may reduce risks of this contamination.
The medium (PDA with added streptomycin) used to quantify the CFUs in each treatment did not sufficiently reduce the background fungi present on the agar plates and made an accurate quantification difficult. A more suitable medium that reduces background bacterial and fungal growth, while not inhibiting Fov CFUs, is thus required.
Treatment was seen to affect germination; however, the germination of Siokra 1-4 was unexpectedly low across all treatments, including the control. The unnaturally high Fov concentrations in treatments 4 and 5 were observed to cause unreliable and inconsistent germination. These treatments also brought about inconsistent vascular browning and stunting symptoms, and did not correlate with cultivar resistances observed in the field or with those reported in previous studies. In treatments 4 and 5 the disease index fluctuated within the genotypes, and surprisingly, the higher CFU densities of these treatments appeared to induce less disease severity, on average, than treatments 2 and 3. These inconsistencies may be due to possible Fov induced root damage.
Treatments 2 and 3 produced the most consistent disease symptoms which correlated strongly with cultivar resistances observed in the field. Germination was shown to be unaffected by the CFU density in treatment 2, which suggests that this level of disease pressure may be most suitable for screening cotton plants in the aid of genetic studies investigating Fov resistance. In treatment 3, however, germination was significantly affected without the plants being overwhelmed by the CFU level. For studies focusing on the effects of pathogen CFU levels on germination, this level of disease pressure may be more valuable.
The soil amended, Fov colonised wheat inoculation method was found to allow an accurate screening of a large number of accessions, and proved to be temporally and spatially efficient. It also permits the comparison of resistance in native and cultivated cottons. Moreover, while refinement of this technique is needed, it may be a more conducive method for producing uniquely pathogen induced responses in plants, as it avoids some of the problems associated with other inoculation methods; some of which include deliberate or unavoidable mechanical damage, the possibility of plants escaping infection during inoculation and certain other factors that can alter the plant’s response to the disease.
The infection process
In field soil, overwintering chlamydospores are the major propagules; whereas in the glasshouse, disease may be a result of microconidia, macroconidia and chlamydospores. (Elmer 2002) The infection process in the field begins with Fov chlamydospores being stimulated to germinate by root exudates secreted by developing plants. The fungus comes in contact with the root system of the cotton plant by producing germ-tubes that grow through the soil. It then typically enters the vascular system of the cotton plant through the roots. (Kochman – Living with Fusarium Wilt; Harrison and Beckman 1982)
First the conidia colonise the root surface and produce a dense net-like mycelium. 24h later the fungus starts to penetrate. Surface hyphae produce branches which penetrate the root inter- and intra-cellularly. The production of penetration hyphae is highest in the meristematic zone, 40% less of that are produced in the elongation and hair root zone, but along the lateral root zone there is no fungal penetration. The exudates that the root secretes through its root cap and meristematic zone may influence both the germination of conidia and the growth of the hyphae. The concentration of these exudates decreases with distance from the root cap and may be a reason for the decrease in the production penetration hyphae in other zones. Differently, the rhizodermis of the lateral root zones of cotton contains high concentrations of terpenoid gossypol, which is suspected to prevent the penetration by the fungus. (Rodriguez-Galvez and Mendgen 1995)
After the fungus has entered the root system it invades the vascular system where the conidia are formed and distributed through out the plant. The plant responses to this invasion with an occlusion-toxin mechanism. It forms a physical barrier; plugging its xylem vessels with gels or gums to restrict the movement of water as well as the pathogen. (Bugdee 1969) This process of occlusion is followed by the formation of toxin in the occluded cells. The toxin heavily suppresses germination of the conidia and contributes to containing the fungus. However, as a result of the fungal invasion the plant can become starved of water, which leads to leaf necrosis and stunted growth, and even to leaf and stem death. The vascular tissue is also affected and becomes discoloured. (Kochman – Living with Fusarium Wilt) This discolouration is most likely due to a wall matrix, with staining properties, which the plant surrounds the fungus with. Vascular discolouration thus indicates the extent of systemic invasion. (Rodriguez-Galvez E and Mendgen K 1995)
Resistant plants respond better to fungal invasions than susceptible plants. It has been found that one of the characteristics of a more resistant plant is its ability to produce xylem tissue at a higher rate than a susceptible plant. The more extensive vascular system of a resistant plant allows it to have a sufficient quantity of functional vessels remaining after the fungus has finally been walled off, and thus the plant can still move water to its leaves. (Bugdee 1969)
Inoculation methods
Stem injection
Inoculation by stem injection usually consists of puncturing the hypocotyl of a 4-week-old cotton plant and administering a drop of suspended conidia at a concentration of 3 - 27 x 106 conidia/ml. Normally an injection is applied to three sites on the hypocotyl, 1.5 – 3.0 cm above the soil line. (Bugdee and Sappenfield 1967; Bugdee 1969)
Root inoculation
To inoculate the roots of cotton plants, this technique involves severing the tap roots of 2-week-old seedlings that are grown in bottomless flint glass tubes and placing them in a conidial suspension for 30 – 45 seconds. (Bugdee and Sappenfield 1967)
Soil infestation technique
Using suspended conidia to infest the soil in which cotton plants are grown can be done by simply pouring the conidial suspension over the surface of the soil or by pouring it into the soil. This method is used in order to cause minimal root damage.
To ensure their conidial suspension entered the soil, Katasantonis, Hillcocks and Gowen planted three cotton seedlings per pot and placed bamboo sticks (1 x 15 cm) next to them, as well as putting a universal bottle (2.5 x 8 cm) in the middle of each pot. All were placed 7 cm deep in the soil. When the plants were 15-days-old, the sticks and bottle were removed and the inoculum (4 x 106 conidia/ml) was poured into the holes. (Katasantonis, Hillocks and Gowen 2003)
Root dipping
Inoculating cotton plants with Fov using the technique termed "root dipping" usually involves carefully removing the plants at an age of 14 days from the soil they are sown or planted into, washing their roots and then dipping them in an inoculum with a concentration that can be from 5 x 106 to 107 conidia/ml for 5 – 20 min. This is followed by re-potting the plants in fresh potting medium. (Wang, Dale and Kochman 1999; McFadden, Beasley, and Brubaker 2004)
Multiple Comparisons ANOVA of Germination
Mean average germination comparison by cotton variety
|
Multiple Comparisons |
|
|
|
|
|
|
|
|
|
LSD |
|
|
|
|
|
|
|
|
|
|
|
Mean Difference (I-J) |
|
Std. Error |
Sig. |
95% Confidence Interval |
|
|
Dependent Variable |
(I) Lines |
(J) Lines |
|
|
|
|
Lower Bound |
Upper Bound |
|
Germination |
Siokra 1-4 |
Sicot 189 |
-1.32 |
* |
0.64 |
0.04 |
-2.59 |
-0.05 |
|
|
Sicot F1 |
-1.96 |
* |
0.64 |
0.00 |
-3.23 |
-0.69 |
|
|
Gos-5050 |
-8.08 |
* |
0.64 |
0.00 |
-9.35 |
-6.81 |
|
|
Gos-5250 |
-8.00 |
* |
0.64 |
0.00 |
-9.27 |
-6.73 |
|
Sicot 189 |
Sicot F1 |
-0.64 |
|
0.64 |
0.32 |
-1.91 |
0.63 |
|
|
Gos-5050 |
-6.76 |
* |
0.64 |
0.00 |
-8.03 |
-5.49 |
|
|
Gos-5250 |
-6.68 |
* |
0.64 |
0.00 |
-7.95 |
-5.41 |
|
Sicot F1 |
Gos-5050 |
-6.12 |
* |
0.64 |
0.00 |
-7.39 |
-4.85 |
|
|
Gos-5250 |
-6.04 |
* |
0.64 |
0.00 |
-7.31 |
-4.77 |
|
Gos-5050 |
Gos-5250 |
0.08 |
|
0.64 |
0.90 |
-1.19 |
1.35 |
|
|
|
|
|
|
|
|
|
Multiple Comparisons ANOVA of Vascular Browning
Mean average vascular browning score comparison by treatment
|
Multiple Comparisons |
|
|
|
|
|
|
|
|
|
LSD |
|
|
|
|
|
|
|
|
|
|
|
Mean Difference (I-J) |
|
Std. Error |
Sig. |
95% Confidence Interval |
|
|
Dependent Variable |
(I) Treatment |
(J) Treatment |
|
|
|
|
Lower Bound |
Upper Bound |
|
Vascular browning |
Control |
5 x 104 |
-2.85 |
* |
0.43 |
0.00 |
-3.70 |
-2.00 |
|
|
1 x105 |
-2.78 |
* |
0.44 |
0.00 |
-3.65 |
-1.92 |
|
|
5 x 105 |
-2.18 |
* |
0.43 |
0.00 |
-3.04 |
-1.32 |
|
|
1 x 106 |
-1.50 |
* |
0.43 |
0.00 |
-2.35 |
-0.65 |
|
5 x 104 |
1 x105 |
0.07 |
|
0.44 |
0.88 |
-0.80 |
0.93 |
|
|
5 x 105 |
0.67 |
|
0.43 |
0.12 |
-0.19 |
1.53 |
|
|
1 x 106 |
1.35 |
* |
0.43 |
0.00 |
0.50 |
2.20 |
|
1 x105 |
5 x 105 |
0.60 |
|
0.44 |
0.18 |
-0.27 |
1.48 |
|
|
1 x 106 |
1.28 |
* |
0.44 |
0.00 |
0.42 |
2.15 |
|
5 x 105 |
1 x 106 |
0.68 |
|
0.43 |
0.12 |
-0.18 |
1.54 |
Note: Treatment 6 was emitted from the ANOVA as very little data could be collected from it.
B. Mean average vascular browning score comparison by cotton variety
|
Multiple Comparisons |
|
|
|
|
|
|
|
|
|
LSD |
|
|
|
|
|
|
|
|
|
|
|
Mean Difference (I-J) |
|
Std. Error |
Sig. |
95% Confidence Interval |
|
|
Dependent Variable |
(I) Lines |
(J) Lines |
|
|
|
|
Lower Bound |
Upper Bound |
|
Vascular browning |
Siokra 1-4 |
Sicot 189 |
1.11 |
* |
0.42 |
0.01 |
0.28 |
1.95 |
|
|
Sicot F1 |
2.19 |
* |
0.43 |
0.00 |
1.35 |
3.03 |
|
|
Gos-5050 |
1.79 |
* |
0.43 |
0.00 |
0.95 |
2.63 |
|
|
Gos-5250 |
-0.79 |
|
0.42 |
0.06 |
-1.62 |
0.05 |
|
Sicot 189 |
Sicot F1 |
1.08 |
* |
0.42 |
0.01 |
0.24 |
1.91 |
|
|
Gos-5050 |
0.67 |
|
0.42 |
0.11 |
-0.16 |
1.51 |
|
|
Gos-5250 |
-1.90 |
* |
0.42 |
0.00 |
-2.73 |
-1.08 |
|
Sicot F1 |
Gos-5050 |
-0.40 |
|
0.43 |
0.35 |
-1.25 |
0.44 |
|
|
Gos-5250 |
-2.98 |
* |
0.42 |
0.00 |
-3.81 |
-2.14 |
|
Gos-5050 |
Gos-5250 |
-2.58 |
* |
0.42 |
0.00 |
-3.41 |
-1.74 |
Multiple Comparisons ANOVA of Average Height
A. Mean average height comparison by treatment
|
Multiple Comparisons |
|
|
|
|
|
|
|
|
|
LSD |
|
|
|
|
|
|
|
|
|
|
|
Mean Difference (I-J) |
|
Std. Error |
Sig. |
95% Confidence Interval |
|
|
Dependent Variable |
(I) Treatment |
(J) Treatment |
|
|
|
|
Lower Bound |
Upper Bound |
|
Average Height |
Control |
5 x 104 |
5.01 |
* |
0.88 |
0.00 |
3.27 |
6.75 |
|
|
1 x105 |
5.47 |
* |
0.88 |
0.00 |
3.73 |
7.20 |
|
|
5 x 105 |
3.64 |
* |
0.88 |
0.00 |
1.90 |
5.37 |
|
|
1 x 106 |
3.67 |
* |
0.88 |
0.00 |
1.93 |
5.40 |
|
5 x 104 |
1 x105 |
0.46 |
|
0.88 |
0.60 |
-1.28 |
2.19 |
|
|
5 x 105 |
-1.38 |
|
0.88 |
0.12 |
-3.11 |
0.36 |
|
|
1 x 106 |
-1.34 |
|
0.88 |
0.13 |
-3.08 |
0.39 |
|
1 x105 |
5 x 105 |
-1.83 |
* |
0.88 |
0.04 |
-3.57 |
-0.09 |
|
|
1 x 106 |
-1.80 |
* |
0.88 |
0.04 |
-3.54 |
-0.06 |
|
5 x 105 |
1 x 106 |
0.03 |
|
0.88 |
0.97 |
-1.71 |
1.77 |
Note: Treatment 6 was emitted from the ANOVA as very little data could be collected from it.
B. Mean average height comparison by cotton variety
|
Multiple Comparisons |
|
|
|
|
|
|
|
|
|
LSD |
|
|
|
|
|
|
|
|
|
|
|
Mean Difference (I-J) |
|
Std. Error |
Sig. |
95% Confidence Interval |
|
|
Dependent Variable |
(I) Lines |
(J) Lines |
|
|
|
|
Lower Bound |
Upper Bound |
|
Average Height |
Siokra 1-4 |
Sicot 189 |
-1.30 |
|
0.80 |
0.11 |
-2.88 |
0.28 |
|
|
Sicot F1 |
-2.22 |
* |
0.80 |
0.01 |
-3.80 |
-0.64 |
|
|
Gos-5050 |
1.88 |
* |
0.80 |
0.02 |
0.30 |
3.45 |
|
|
Gos-5250 |
4.20 |
* |
0.80 |
0.00 |
2.62 |
5.78 |
|
Sicot 189 |
Sicot F1 |
-0.92 |
|
0.80 |
0.25 |
-2.49 |
0.66 |
|
|
Gos-5050 |
3.18 |
* |
0.80 |
0.00 |
1.60 |
4.76 |
|
|
Gos-5250 |
5.50 |
* |
0.80 |
0.00 |
3.92 |
7.08 |
|
Sicot F1 |
Gos-5050 |
4.09 |
* |
0.80 |
0.00 |
2.51 |
5.67 |
|
|
Gos-5250 |
6.42 |
* |
0.80 |
0.00 |
4.84 |
8.00 |
|
Gos-5050 |
Gos-5250 |
2.32 |
* |
0.80 |
0.00 |
0.74 |
3.90 |
Medium for CFU counts:
Komada’s selective medium
(Komada 1975)Komada reports that on his medium F. oxysporum grows rapidly, bacterial development is completed inhibited and fungal contaminates are suppressed. The few colonies of fungal contaminates that develop are very small and do not interfere with the identification and counting of F. oxysporum colonies. With this medium it is possible to estimate F.oxysporum density in mildly infested soil. He found disease incidence to highly correlate with the pathogen population density that was estimated from dilution plates using his selective medium.
Formulation and preparation:
Basal medium
|
K2HPO4 |
1.0 g |
|
|
KCl |
0.5 g |
|
|
MgSO4.7H2O |
0.5 g |
|
|
Fe-Na-EDTA |
0.01 g |
|
|
L-Asparagine |
2.0 g |
|
|
D-Galactose |
20.0 g |
|
|
Water |
1000 ml |
After the basal medium is melted and cooled the antimicrobial supplement is added and mixed thoroughly.
Antimicrobial supplement
|
PCNB (75% WP) |
1.0 g |
|
|
Oxgall |
0.5 g |
|
|
Na2B4O7.10H2O |
1.0 g |
|
|
Streptomycin sulfate |
0.3 g |
This is to be followed by adjusting pH.
Adjustment of pH
The medium’s hydrogen-ion concentration is adjusted to pH 3.8±0.2 with approximately 10% solution of phosphoric acid.
15ml of the medium is poured into a 9cm diameter petri dish and, when using the dilution plate method, 0.5 ml (or 1 ml) of the soil suspension should be evenly spread over the surface. It is not necessary to incubate the plates under a strictly controlled temperature (25 – 30ºC). Incubation (for a period of up to 7 days) under diffuse daylight increases pigmentation and development of aerial hyphae of F. oxysporum, facilitating its identification.
The mechanisms of selectivity for F. oxysporum:
Streptomycin or oxgall, in addition to acidification, selectively inhibit and completely suppress the development of bacteria and actinomycetes. Fungal contaminants are suppressed by using D-galactose as a sole carbon source and L- asparagine as a sole nitrogen source. L-Asparagine and acidification allow F. oxysporum to develop pigmentation, and D-galactose suppresses the development of fungal contaminants but supports the development of F. oxysporum.
Fov induced root damage in high conidia concentrations
The disease incidence of the plants in treatments 4 and 5 appeared to be lower than those in the treatments of lower conidial concentrations. They had lower vascular browning scores and had higher growth rates than those in treatments 2 and 3. It was observed, however, that the plants in treatments 4 and 5 had thicker leaves of waxy appearance, and their roots were shallow and appeared damaged.
Rodriguez-Galvez and Mendgen report that the number of conidia used for inoculation positively correlates with the density of fungal penetration hyphae present on plant roots. (Rodriguez-Galvez and Mendgen 1995) The conidial concentrations used in treatment 4 (5 x 105 conidia/g) and treatment 5 (1 x 106 conidia/g) are well above levels observed in infested fields (5 x 104 conidia/g). (Australian Cotton CRC) These artificially high conidial concentrations, according to what has been reported by Rodriguez-Galvez and Mendgen, will correlate with artificially high densities of penetration hyphae on the roots of the plants in those treatments. Such high densities of penetration hyphae can be expected to severely damage colonised roots. Since penetration hyphae densities are greatest along the meristematic and elongation zone of the root system, but absent along the lateral root zone (Rodriguez-Galvez and Mendgen 1995), only the root tip and elongation zone will be affected and the lateral roots will remain intact.
The plants in treatments 4 and 5 had low vascular discolouration and displayed shallow but branched root systems. Being highly possible that the observed root damage was Fov induced, only the lateral roots of these plants remained functional. With only lateral roots left, the plants lacked sufficient water and responded normally by growing thicker, waxier leaves in order to conserve water. Furthermore, as the root zones of these plants that are susceptible to penetration hyphae were no longer functional, the Fov would have had no way of entering their vascular systems, leaving them relatively disease free in appearance.
REVIEW OF INNOCULATION METHODS
"Every refutation should be regarded as a great success; not merely a success of the scientist who refuted the theory, but also of the scientist who created the refuted theory and who thus in the first instance suggested, if only indirectly, the refuting experiment." – Karl Popper
Many methods of inoculating plants with Fusarium in the glasshouse have been employed in the past, and, attempts are being made to refine them with the aim of developing a more efficient and accurate technique. Inoculation in the glasshouse is used both to test the pathogenicity of different Fov isolates, and to screen cotton varieties for Fov resistance. The infection process involves the interaction between the fungus and the plant. Therefore, the characteristics and properties of both the fungus and the plant play an important role in this process and need to be taken into account when developing an inoculation method.
Kochman reports that Fov is a stress-related pathogen, which means that stress affects the resistance that cotton plants normally have to the disease and renders them more susceptible. (Kochman, Swan, Moore, et al. 2002) The plant’s response to the fungus can be altered by a number of factors: various forms of mechanical damage inflicted upon the plant, the area of the plant to which the inoculum is applied, the conidial concentration of the inoculum, the age at which the plant is inoculated, and the length of time the plant is in contact with the inoculum. In addition, the physical properties of the fungus can complicate certain inoculation techniques.
Trials run on inoculation by stem injection have shown that the number of injections a plant receives into its stem affects the severity of wilting symptoms which subsequently develop. Bugdee and Sappenfield compared the wilt in plants that were treated with the same total concentration of conidia (in total 25 x 106 conidia/ml per plant) administered wholly, by one injection, then partially by two and three injections per stem respectively. They report that plants administered with a single injection showed signs of developing wilt symptoms, but severe wilt did not develop and most plants recovered. However, they also found that among plants receiving multiple injections wilt prevalence was more uniform and disease severity increased as the number of injections increased. They suggest that this supports the possibility that more xylem elements are infected as the number of injections per stem increases. (Bugdee and Sappenfield 1967) Nevertheless, they do not include in their discussion an analysis of the level of mechanical damage, as a result of multiple stem injections, inflicted upon the plant and the corresponding increased stress level to which the plant is inflicted. The increased level of stress has the effect of increasingly slowing the plant’s rate of response to the pathogen thereby causing greater systemic invasion. Budgee and Sappenfield’s results roughly correlate with field observations, however examples were found of cultivars showing more, and some times less, resistance in the glasshouse than in the field.
Bugdee and Sappenfield simultaneously conducted a trial on a root inoculation method to compare with the stem inoculation method. This root inoculation method included inflicting deliberate mechanical damage on the plant, which consisted in severing the tap roots of cotton seedlings prior to dipping them in the inoculum. They found the effect of this mechanical damage to be that non-inoculated plants with severed roots had stunted growth compared to non-treated plants. When comparing the results of both inoculation methods they found that the differential response among varieties was unchanged; whereas the wilt reaction was more severe and the symptoms began earlier on plants inoculated in the stem. (Bugdee and Sappenfield 1967) This suggests that while cotton plants display resistance in both the stem and the roots, the roots respond better to infection and mechanically induced stress than the stem.
Katsantonis, Hillocks and Gowen ran an experiment to test the effect of root-knot nematodes on Fusarium wilt in cotton using two different inoculation methods: inoculation through infested soil, using a fungal suspension of 4 x 106 spores/ml, and inoculation by stem injection where the plants were injected twice with a drop of the same fungal suspension. When comparing the results from both inoculation methods, conducted under root-knot nematode free conditions (as well as in the presence of root-knot nematodes), the resistance displayed by some cultivars changed dramatically under the influence of the different techniques. (Katsantonis et al. 2003)
Without covering the differences in the plant’s response when inoculated at the stem or the roots again, the inconsistencies seen between the two techniques may have been due, firstly, to the dramatically different inoculum loads to which the plants would have been exposed; despite of the same fungal suspension being used for both. The volume of the fungal suspension used for the soil infestation technique was much greater than that used for the stem injections. Secondly, the plants inoculated by stem injection would have been under more stress from mechanical damage than those grown in the infested soil which received no deliberate mechanical damage.
The two inoculation methods are very different and are complicated by different factors. For the soil infestation technique, the characteristics of spore movement through the soil would determine the percentage of the plant’s root system exposed to the Fov and would affect the amount of inoculum the plants would take up. Gracia-Garza and Fravel report that the size, shape and electrical charge of spores, as well as the physical properties of the soil such as the pore size and electrical charge of the soil particles, are assumed to affect the dispersal of fungal propagules in soil water. They found after placing a sporulating formulation of Fusarium oxysporum f.sp. niveum on soil columns, and applying simulated rain to entirely wet the soil, that nearly all propagules were retained within the upper 2 cm. Continuing their discussion by drawing on conclusions made in studies previous to theirs, Gracia-Garza and Fravel inform us that propagules of Fusarium are rapidly filtered out as water percolates through soil, and that the low rate of passive transport of some spores within the soil may be accounted for by their hydrophobic nature. (Gracia-Garza and Fravel 1998) Considering the soil infestation technique in light of this, the fungal suspension poured into the soil would not disperse much and would remain in concentrated pockets. Since the inoculum was only poured into a hole on one side of each plant, it can be assumed that the roots of the plants were not uniformly exposed to the Fov. If in majority only the lateral roots were exposed to the inoculum then the extent of infection would be different than if only the tap root was exposed. Moreover, such highly concentrated pockets of conidia could have adverse effects on the plant and result misleading disease symptoms.
Currently, the most commonly used method of inoculation is root dipping. Studies conducted using this technique have shown that the concentration of the conidial suspensions used, the inoculation time and the age of the plant when inoculated can all alter disease severity. It was found that as conidial concentration increases, so does the disease index; that as the inoculation time increases (from 1 to 25 min), so also does the disease index; and that plants inoculated at 1 week of age always show a higher degree of disease symptoms than those inoculated later, regardless of the cultivar. (Wang, Dale and Kochman 1999)
When testing for the minimum concentration of inoculum required to cause disease symptoms in cotton plants using the root dipping technique, Wang reported that the susceptible cultivar, Siokra 1-4, showed no signs of disease when conidial concentrations were below 1.0 (± 0.2) x 105 conidia/ml, and that a concentration of 1.0 (± 0.2) x 106 conidia/ml was required before the resistant cultivar CS 189+ showed symptoms. In the field, however, soils containing 5 x 104 spores/g can cause disastrous wilting of cotton crops. (Australian Cotton CRC) Using a soil amended, Fov colonised wheat substrate to inoculate cotton plants, conidia levels of ± 1000 conidia/g were observed to cause disease symptoms in both susceptible and resistant cultivars. (This report) Similar low levels of Fusarium oxysporum f. sp. cyclaminis (50 to 500 CFU/ml) in field soils have been reported to cause severe disease in cyclamens. The symptoms involved in the Fusarium wilt of cyclamens are similar to those displayed by cotton when infected by Fov. They include chlorosis, stunting and wilting, as well as a characteristic reddish-brown discolouration in the vascular tissue. Elmer found, when using a soil amended colonised substrate to inoculate cyclamens, that consistent vascular discolouration became apparent when inoculated soil levels reached 5.4 x 104 CFU/ml. He used inoculum densities of 6.2 x 104 CFU/ml of potting mix to provide a consistent level of disease pressure without overwhelming the plant. (Elmer 2002) Again, using a very similar technique to inoculate cotton plants, similar levels of Fov in potting mix were observed to bring about consistent vascular decolouration without overwhelming the cotton plants. (This report)
The discrepancies between the minimum concentrations of inoculum needed for disease symptoms to appear, when using the two techniques, may be due to the length of time the plants are exposed to the inoculum in each method. Cotton plants grown in potting mix inoculated with a soil amended colonised substrate are in constant contact with the Fov, and since the dry inoculum is evenly mixed through the soil (avoiding the problems incurred when infesting soil with liquid inoculum) the inoculum load is uniform through out the soil, which means the probability for plants to escape inoculation is very low. Plants inoculated by root dipping, however, are exposed to the inoculum for only a matter of minutes (5 – 25 mins). As mentioned earlier, disease severity is a function of the inoculation time and the concentration of inocula. (Wang, Dale and Kochman 1999) Therefore, the higher minimum inoculum concentrations required to induce disease symptoms using this method may be the result of a need to compensate for the small amount of conidia the roots take up during the very short inoculation time. The disease pressure on the plants when using this method is therefore undetermined, as there is no way of verifying the amount of conidia a plant has had the opportunity to take up. The resulting disease pressure may also vary from plant to plant. This implies that the severity of disease symptoms displayed by a plant may, in equal measure, be due to the plant’s natural level of susceptibility to infection, or the dose of conidia taken up by the plant. The conidial concentrations used for this method of inoculation are capable of overwhelming even the most resistant plant.
Another constraint of the root dipping technique involves the age at which a plant can be inoculated. Wang informs us that it is important to inoculate plants at their most susceptible stage, which is at one week of age. (Wang, Dale and Kochman 1999) However, because of the unavoidable mechanical damage that the roots incur as a result of this technique, it was found that 2-week-old seedlings were the most suitable plants for inoculation. These plants have relatively high and stable susceptibility, and well a developed root system which allows them to withstand better the shock of being removed from the soil, inoculated and then re-planted. Moreover, they are easier to handle at that age. The constraints of this technique do not easily allow seedlings to be inoculated at the optimal age.
The optimal glasshouse inoculation technique for both testing the pathogenicity of a fungus and screening plants for resistance is one that will obtain a disease index that is uniquely disease induced. Developing this inoculation technique involves eliminating non-pathogen related stresses on the plant, and employing a method that is conducive to the physical properties and characteristics of both the plant and the fungus itself. Furthermore, the disease pressure needs to be equal and consistent for all plants being tested, and the inoculum used should be at a concentration that will produce consistent symptoms without adversely affecting the infection process.
Thanks to:
The Australian Pastoral Research Trust for providing the summer student scholarships.
Cotton CRDC for providing plant materials.
Dr. Bo Wang (CSIRO Plant Industry) for technical consultations.
Dr. Celeste Linde (Australian National University) for support with identification of soil fungi.
Dr. Bob Godfree (CSIRO Plant Industry) for ecological field trip.
Walter Tate (CSIRO Plant Industry) for technical support.
Matthew Woods (CSIRO Plant Industry) for technical support.
Australian Cotton CRC (Cotton Catchment Communities) (2005) Integrated Disease Management for Fusarium Wilt. www.cotton.pi.csiro.au/Assets/PDFFiles/Disease/IDMGL02.pdf
Bell A.A., Wheeler M.H., Lui J., et. al. (2003) United Staes Department of Agriculture – Agricultural Research Service studies on polyketide toxins of Fusarium oxysporum f sp vasinfectum: potential targets for disease control. Pest Management Science, 59: 739-747.
Bugdee W. M. (1969) Vascular Response of Cotton to Infection by Fusarium oxysporum f.sp.vasinfectum. Phytopathogy, 60: 121-123.
Bugdee W. M. and Sappenfield W. P. (1967) Varietal Reaction of Cotton after Stem and Root Inoculation with Fusarium oxysporum f.sp. vasinfectum. Phytopathology, 58: 212-214.
Elmer WH (2002) Influence of Inoculum Density of Fusarium oxysporum f. sp. cyclaminis and Sodium Chloride on Cyclamen and the Development of Fusarium Wilt. Plant Disease, 86 (4): 389-393.
Gracia-Garza JA and Fravel DR (1998) Effect of Relative Humidity on Sporulation of Fusarium oxysporum in Various Formulations and Effect of Water on Spore Movement through Soil. Phytopathology, 88(6): 544-549.
Harrison N.A. and Beckman C.H. (1982) Time/space relationships of colonisation and host response in wilt-resistant and wilt-susceptible cotton (Gossypium) cultivars inoculated with Verticillium dahliae and Fusarium oxysporum f.sp.vasinfectum. Physiological Plant Pathology, 21: 193-207.
Katasantonis D, Hillocks RJ and Gowen S (2003) Comparative Effect of Root-knot Nematode on Severity of Verticillium and Fusarium Wilt in Cotton. Phytoparasitica 31 (2).
Kochman J, Swan L, Moore N, et al. The Fusarium threat – are we making the progress? From the proceedings of the 11th Cotton Conference, 13th -15th August 2002. cotton.pi.csiro.au/Publicat/conf/coconf02/soilhelt/088/088.htm
Kockman J (??) Living with Fusarium.
Komada, H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Review of Plant Protection Research, 8: 114-124.
McFadden H., Beasley D., and Brubaker C.L. (2004) Assesment of Gossypium sturtianum and G australe as potential sources of Fusarium wilt resistance to cotton. Euphytica, 138: 61-72.
Rodriguez-Galvez E. and Mendgen K. (1995) The infection process of Fusarium oxsporum in cotton root tips. Protoplasma, 189: 61-72.
Wang B, Brubaker CL and Burdon J (2004) Fusarium species and Fusarium wilt pathogens associated with native Gossypium populations in Australia. Mycol. Res. 108 (1): 33-44.
Wang B, Dale ML and Kochman (1999) Studies on a pathogenicity assay for screening cotton germplasms for resistance to Fusarium oxysporum f.sp.vasinfectum in the glasshouse. Australian Journal of Experimental Agriculture, 39: 967-974.