Reproduction & dispersal
Sexual vs. vegetative
The sections on SEXUAL REPRODUCTION and VEGETATIVE REPRODUCTION have shown various ways in which sexual and vegetative reproduction can occur in the bryophytes. This page will compare the two modes of reproduction. It's not the case that one is better than the other. In bryophytes which reproduce in both ways (and all bryophytes can reproduce vegetatively), it is likely that the two methods play different roles in dispersal. There are circumstances in which sexual reproduction is impossible or very unlikely. In some cases conditions may be too harsh for sexual reproduction – perhaps too hot and dry, with free water extremely rare, or perhaps (in arctic or alpine areas) with growing seasons too short to allow the cycle of gametophyte-sporophyte stages. It has also been proposed that during events in the distant past (such as Pleistocene glaciations) some bryophyte populations were stressed and fragmented, leaving isolated pockets where once there was extensive cover. After such events some of the surviving, isolated pockets may have been entirely female, others entirely male. For such isolated populations, continued survival (perhaps even until the present day) would have been possible only by vegetative means. There's more discussion in the BARRIERS TO SEXUAL REPRODUCTION CASE STUDY. The GENETIC DIVERSITY CASE STUDY looks a little at the roles of sexual and vegetative reproduction in promoting genetic diversity![]() .
.
Roles in dispersal
The spores in most bryophytes are quite small and can easily be carried considerable distances by breezes. Some calculations suggest that spores with diameters between 8 and 12 micrometres are capable of being carried over 19,000 kilometres. One micrometre is a thousandth of a millimetre. The larger the spore the harder it is to move by wind. Corresponding calculations for spores with diameters of about 30 micrometres suggest they could not be wind-dispersed much beyond 300 kilometres![]() .
.
Such theoretical calculations give food for thought but don't prove that the majority of small spores travel great distances. In fact several studies have shown that many spores land relatively close, even within a few centimetres, to the sporophyte that produced them. Neither do those theoretical calculations rule out the possibility of larger spores being carried much further away in some circumstances. As the LONG DISTANCE CASE STUDY shows, long distance spore dispersal does occur but, in order for it to occur spores must reach high latitudes and be able to survive various dangers if they are to have a chance of germinating thousands of kilometres away.
Given the obstacles and dangers, only a small proportion of viable spores are likely to land far away. However, some species produce prodigious numbers of spores, so that even a small proportion may translate to a large number, thereby giving spores a role as agents of long distance dispersal. Here are some estimates of the total number of spores produced annually, per square metre of moss cover, for several moss species.
Name |
Annual spores/sq m |
Distribution |
Atrichum undulatum |
100 million to 400 million |
widespread; Northern Hemisphere |
Bryum argenteum |
3,700 million |
cosmopolitan |
Grimmia pulvinata |
9,300 million |
cosmopolitan |
Tortula muralis |
8,500 million to 38,000 million |
cosmopolitan |
Polytrichum alpestre |
5,600 million |
widespread |
The estimates come from a study done in the United Kingdom, but these species are found in many places outside the UK, as indicated in the rightmost column. Three of the five are found in Australia: Bryum argenteum, Grimmia pulvinata and Tortula muralis. Bryum argenteum is a very common silvery green to greyish green moss found in exposed areas. Here is a picture of a very small colony (only a few square centimetres in size) growing in the middle of the gravelled parade area along the middle of Anzac Parade, leading up to the War Memorial in Canberra. Here is a picture ![]() of part of a large colony, several square metres in size growing on an old rabbit warren in Black Mountain Nature Reserve in Canberra. In Canberra this species is very common along suburban roads, in the gaps between the concrete gutters and the bitumen. Grimmia pulvinata grows in cushion form on boulders and Tortula muralis can be found growing on soil but is also very common on stone or brick walls. All three species grow in the grounds of the Australian National Botanic Gardens.
of part of a large colony, several square metres in size growing on an old rabbit warren in Black Mountain Nature Reserve in Canberra. In Canberra this species is very common along suburban roads, in the gaps between the concrete gutters and the bitumen. Grimmia pulvinata grows in cushion form on boulders and Tortula muralis can be found growing on soil but is also very common on stone or brick walls. All three species grow in the grounds of the Australian National Botanic Gardens.
By contrast, vegetative methods are less likely to be methods of long distance dispersal. In many cases dispersal by vegetative means is measured in terms of centimetres. This is clearly the case in a moss such as Octoblepharum albidum, which produces small plantlets at leaf tips. These plantlets eventually become independent plants, growing very close to their parents. Bryophyte fragments and gemmae, though sometimes quite small, are often too large for easy, long distance wind dispersal. Moreover, with regard to gemmae at least, it has been noted that, in many cases, they would not withstand drying and so would be unsuited to long distance wind dispersal. Overall the role of vegetative reproduction methods is local spread of the bryophyte in question – or simply maintaining an existing colony. Vegetative means can be quite effective in increasing the local territory occupied by a bryophyte colony. An example of this, in a very ordinary setting, involves the spread of the moss Campylopus clavatus along a garden path in the suburb of Macquarie in Canberra. The path in question is about a half metre wide, about six metres long, is composed of a compacted mix of course sand and small eucalypt chips and is on a gentle slope. A small colony of Campylopus clavatus first became noticeable at the upper end of this path about a year after the path had been made. At that time the moss colony was a few square centimetres in size and was partially obscured by a nearby grass tussock. There were no other bryophytes on the path at the time and the Campylopus colony had abundant vegetative propagules, in the form of broken stem tips, lying loose on its surface. Such propagules are common in this species, as shown by this photo ![]() . Within a decade after the first sight of the moss, a large proportion of the path was covered by Campylopus clavatus. It is likely that much of this coverage, if not all, arose from stem tip fragments washed downhill.
. Within a decade after the first sight of the moss, a large proportion of the path was covered by Campylopus clavatus. It is likely that much of this coverage, if not all, arose from stem tip fragments washed downhill.
Naturally there are exceptions to the "spores=long and vegetative propagules=short" dispersal distances. Various mosses in the family Splachnaceae have small spores, but the spores are sticky and clump together, so ruling out wind dispersal. In fact dispersal is by carrion-loving insects. The mosses in question commonly grow on dung or old carcases. The flies and similar insects that pick up the sticky spores are most likely to deposit them on other carcases or animal droppings, not too far away from where the spores were picked up.
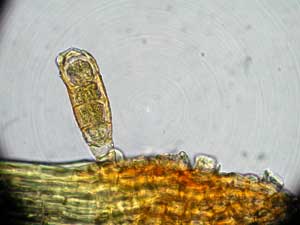
Mosses in the genus Archidium have large spores, up to 200 micrometres (a fifth of a millimetre) in diameter in some species. A number of other moss genera and some liverworts (eg the genus Riccia) have spores with diameters of 50 micrometres or more. In the liverwort Sphaerocarpos texanus ![]() the spores are 15 to 30 micrometres in diameter, but are clumped together in groups of four. Not surprisingly such spores (or aggregates) are very unlikely to be carried appreciable distances by the wind. Water, such as ground flow after rain, could carry such spores fairly easily, but probably not very far in most cases. Stones, twigs, plants or uneven ground are all capable of trapping spores that are being carried on a sheet of surface water. One case where water could carry spores a considerable distance is streamside bryophytes. Spores carried away by the current could easily wash up a long way downstream. In the discussion about Pleurophascum in the DISPERSAL SECTION you will have seen the suggestion that buoyant, air-filled spore capsules could be carried away by water.
the spores are 15 to 30 micrometres in diameter, but are clumped together in groups of four. Not surprisingly such spores (or aggregates) are very unlikely to be carried appreciable distances by the wind. Water, such as ground flow after rain, could carry such spores fairly easily, but probably not very far in most cases. Stones, twigs, plants or uneven ground are all capable of trapping spores that are being carried on a sheet of surface water. One case where water could carry spores a considerable distance is streamside bryophytes. Spores carried away by the current could easily wash up a long way downstream. In the discussion about Pleurophascum in the DISPERSAL SECTION you will have seen the suggestion that buoyant, air-filled spore capsules could be carried away by water.
A number of bryophytes with large spores live in arid areas and some, such as the moss genera Acaulon and Ephemerum, are short-lived. Spores germinate, gametophytes are fertilized and produce sporophytes, which release spores and the n the parent plants all die – all in a relatively short period. In such cases the spores would primarily ensure the continuation of the species at a given location, rather than act as agents of long-distance dispersal. This, and other strategies are covered in more detail in the LIFE STRATEGIES CASE STUDY. Incidentally, there are also short-lived, small-spored arid area bryophytes.
In some circumstances vegetative propagules will move further than spores. An example of this is the case of vegetative propagules dispersed by European wild boar, given in more detail in the DISPERSAL SECTION. In a forest with understorey plants breezes are very unlikely to carry spores any great distance, since there are many obstacles which would trap wind-blown spores. Animals, such as boars, which forage over several kilometres in a day could easily carry vegetative propagules considerable distances. There is the possibility that gemmae have been responsible for bringing the moss Ulota phyallantha to Macquarie Island, in the subantarctic area south of New Zealand. This moss is known from many of the cool, temperate, maritime parts of the Northern Hemisphere but in the Southern Hemisphere the species is known only from Macquarie Island and southern South America. On Macquarie Island it is abundant on the west coast (the side that faces the prevailing winds) but much less common in the east. This distribution would be consistent with gemmae originating in South America and being carried to Macquarie Island on the circum-subantarctic winds. The gemmae are cylindrical, about 25 micrometres in diameter and from about 80 to 150 micrometres long. Despite their large size, it has been argued that the strong circum-subantarctic winds would be capable of carrying the gemmae. The gemmae are coloured brown, perhaps by a pigment that provides protection against the elements and so help ing long distance survival. It is also worth noting that, in both Northern and Southern Hemispheres, sporophytes have very rarely been found, whereas gemmae are abundant. This supports the idea that gemmae are the primary method of reproduction and dispersal. This photo ![]() shows parts of several leaves of Ulota phyllantha. You can see the very prominent nerve in the largest leaf. To the left of that leaf you can see the upper part of another leaf with a brown gemma still attached and protruding at almost a right angle. At the apex of that leaf you can also see some stubs, left when other gemma broke off. The photo (right) shows a closer view of that leaf apex and the still-attached gemma
shows parts of several leaves of Ulota phyllantha. You can see the very prominent nerve in the largest leaf. To the left of that leaf you can see the upper part of another leaf with a brown gemma still attached and protruding at almost a right angle. At the apex of that leaf you can also see some stubs, left when other gemma broke off. The photo (right) shows a closer view of that leaf apex and the still-attached gemma![]() .
.
In summary, while there may be general rules for the dispersal roles of sexual versus vegetative propagules, specific circumstances may change things. Take the case of a particular species of moss that may be found growing in a forest as well as in a grassland. In the forest it may be that animal-dispersed vegetative propagules are the major means of longer distance dispersal, while in the grassland wind-blown spores are the predominant agents of longer distance dispersal. In these two scenarios you could say that by having both vegetative and sexual methods of reproduction, the moss is optimising its chances of spread regardless of what sort of habitat it may find itself in.
The subject of spore or vegetative propagule dispersion is related to the subject of the distribution and dispersal of species, which is discussed in the BRYOGEOGRAPHY SECTION.
Other environmental and habitat influences
The tendency towards sexual or vegetative reproduction can be influenced by genetic and environmental factors. European studies of rhizoidal gemmae have shown that they have a tendency to be produced by mosses growing in frequently disturbed habitats, both those disturbed by nature (e.g. stream banks and steep slopes subject to erosion) and those disturbed by humans (e.g. arable fields)![]() .
.
Various bryologists have observed that, amongst the liverworts, vegetative methods are common in temperate regions but rare in the tropics. The BARRIERS TO SEXUAL REPRODUCTION CASE STUDY reported observations about dioicous mosses (that is those with separate male and female plants) producing sporophytes less frequently than monoicous species.
All those statements don't mean that rhizoidal gemmae are found only in disturbed areas, that a tropical liverwort is incapable of producing abundant gemmae, nor that all dioicous mosses have difficulty producing sporophytes. Those rather simple, broad statements summarise the "more likely" behaviour and give some useful information, but do not express universal truths. For definitive statements about particular species in specific habitats, there's no alternative to careful investigations. The SYNTRICHIA CANINERVIS CASE STUDY shows one such in-depth investigation into how the harsh environment of a Californian desert adds its influence to that of dioicism and tips the balance very much in favour of vegetative reproduction. There are other environments, less extreme than deserts, which also influence behaviour and at local levels. In ephemeral habitats, not necessarily in harsh environments, relatively large vegetative propagules can give a competitive advantage over smaller spores. A larger propagule has a better chance of surviving and growing into a new gametophyte. Therefore, other things being equal, vegetative propagules are likely to increase the chances of a successful occupation of an ephemeral habitat. The TETRAPHIS PELLUCIDA CASE STUDY looks at a moss that employs vegetative means to colonize fresh substrates but where sexual reproduction occurs once there is a dense moss colony.
Here's a summary of a study into colonies of the moss Octoblepharum albidum in Hong Kong, another environment quite different to that of the Californian desert. The authors compared the tendencies towards sexual or vegetative reproduction in two microhabitats.
|
Sunny and dry |
Shaded and moist |
Sexual reproduction |
Frequent |
Rare |
Leaf gemmae |
Rare |
Frequent |
Protonemal gemmae |
Not seen |
Only seen in laboratory |
Leaf-tip plantlets |
Not seen |
Frequent |
The authors of the study noted their findings, but did not suggest any possible causes of the differences they found. There is more about this species, including a description of the production of the leaf-tip plantlets, in the VEGETATIVE REPRODUCTION SECTION![]() .
.
An American bryologist investigated the possible effect of soil type on gemma production in the moss Bryum bicolor (also known by the name Gemmabryum dichotomum). The gemmae in question are produced in the leaf axils on the gametophytes. He collected samples from four sites (two in an urban area and two on mine tailings), ground them to provide vegetative fragments and then grew new gametophytes from those fragments. He found that, regardless of origin, all gametophytes produced more gemmae when grown on pure mine tailings than when grown on pure sand. While the mine tailings acted to promote gemma growth, it was difficult to come up with a good statistical measure of the strength of the influence exerted by mine tailings. He also noted a Spanish finding that urban plants of the moss Tortula pagorum (right) produced more gemmae than did rural plants![]() .
.
Various mosses are known to produce gemmae at the protonemal stage, some species doing so even in very low light intensities. In very low light a protonema may persist indefinitely, never producing the leafy shoots of the gametophyte. In such a situation the production of gemmae still allows the moss to reproduce, even though the production of sexual organs is not possible. Moreover, the dispersed gemmae may land in a better-lit location where full development of the gametophyte is possible. In such a case the gemmae can be viewed as a means of escaping a far from ideal habitat![]() .
.
Timing
In bryophytes which reproduce both sexually and vegetatively, the two methods may be followed concurrently or perhaps under different circumstances.
Simultaneous production of gemmae and sexual organs has been seen in Cololejeunea cardiocarpa This leafy liverwort is essentially a widespread tropical species (being known from the Americas, Africa, New Caledonia and Queensland , Australia) but is also found in some non-tropical areas, for example southern Africa, northern New Zealand offshore islands and north to Virginia in the USA. In such "marginal" areas, further away from the tropics, gemmae may be more common. In such locations even when sexual organs are produced the production of sporophytes may be inhibited, but with enhanced gemma production. Concurrent immature sporophytes and gemmae occur on the leafy liverwort Aphanolejeunea ephemeroides and in some species of Radula, another leafy liverwort genus, gemmae have also been found on sexually mature plants![]() .
.
In some species of the moss genus Pohlia sterile gametophytes produce gemmae in abundance, while gametophytes with sexual organs or with sporophytes have at most few gemmae. In Metzgeria (a genus of thallose liverworts) gemmae are produced at the thallus margins. Gemma production appears to be more common in juvenile stages, before the development of sporophytes. Bryum bicolor, Bryum erythrocarpum and Fissidens cristatus are three of the moss species which produce rhizoidal gemmae. In these three production of such gemmae stops while the sporophyte is developing. It's not clear if this is the case with all, or even the majority, of mosses with rhizoidal gemmae![]() .
.
In conclusion
There is still much that is unknown about the relative contributions of sexual and vegetative reproduction in bryophyte lives. This page has shown you some of the reproductive strategies followed by bryophytes, but the number of detailed studies of such strategies is still rather small. Given the multitude of habitats in which they occur and the range of pressures they experience, it would be very surprising if bryophytes produced no more surprises for us!
See also:
Vegetative Reproduction
Sexual Reproduction
Additional References
Laaka-Lindberg, S. (2000). Ecology of asexual reproduction in hepatics. Publications in Botany from the University of Helsinki, No. 29. (Available online at: http://ethesis.helsinki.fi/julkaisut/mat/ekolo/vk/laaka-lindberg/ecologyo.pdf)
Laaka-Lindberg, S; Korpelainen, H & Pohjamo, M. (2003). Dispersal of asexual propagules in bryophytes. Journal of the Hattori Botanical Laboratory, 93, 319-330.
Longton, RE. (1994). Reproductive biology in bryophytes. The challenge and the opportunities. Journal of the Hattori Botanical Laboratory, 76, 159-172.
Longton, RE & Schuster, RM. (1983). Reproductive Biology. In RM Schuster (ed). New Manual of Bryology. Vol 1, pp 386-462. Hattori Botanical Laboratory, Nichinan, Japan. [A detailed, technical survey of bryophyte reproduction.]
Miles, CJ & Longton, RE. (1990). Role of spores in reproduction of mosses. Botanical Journal of the Linnean Society, 104, 149-173
Newton , AE & Mishler, BD. (1994). The evolutionary significance of asexual reproduction in mosses. Journal of the Hattori Botanical Laboratory, 76, 127-145.
Wyatt, R. (1994). Population genetics of bryophytes in relation to their reproductive biology. Journal of the Hattori Botanical Laboratory, 76, 147-157.
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)