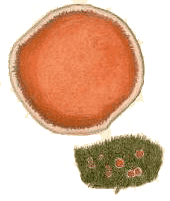
(above) The fungus Cantharellus retirugus (known today as Arrhenia retiruga) growing on moss plants. The illustration is taken from M.J. Berkeley's Outlines of British Fungology, 1860 |
Ecology
Bryophytes & fungi
Fungi can be found growing on or near many bryophytes. The 1988 paper given in the next reference button lists over 300 bryophyte species and the fungi that have been found growing on them – collectively called bryophilous fungi. Not surprisingly, many more bryophyte-fungus associations have been discovered since then and several hundred species of bryophilous fungi are known. A great many of those fungi are obligately bryophilous, which is to say they are found only on bryophytes but there are also the facultatively bryophilous species, which are not restricted to bryophytes![]() .
.
On this page you will see the following fungal terms: apothecium, ascomycete, ascus, basidiomycete, fruiting body, hypha, mycelium, mycorrhiza, perithecium, sclerotium. If you are not familiar with them you'll find explanations in the FUNGAL BASICS CASE STUDY.
The relationships between fungi and bryophytes are varied. There are fungi that are parasitic on bryophytes and with no benefits to the host bryophytes. It is not surprising that many fungi exploit bryophytes as a food source. Wherever there is some organic material, there will be some fungus to exploit it. At the other extreme there is at least one bryophyte that, to put it simply, steals food from other plants via fungi. In between, there are also mycorrhiza-like associations between fungi and some liverworts. While much has been discovered, there are still many bryophyte-fungus pairs where the nature of the association is unknown. Of necessity the number of species mentioned as illustrative examples will be very small but they will be enough to let you see a good range of bryophyte-fungus relationships![]() .
.
Physical proximity doesn’t always imply an intimate connection so we'll look first at...
Coincidental associations
These mushrooms in the genus Galerina are showing above a bryophyte carpet that's covering much of a rotting log in a wet sclerophyll forest near Canberra. The fungal mycelium is within the rotting log and in this case the juxtaposition of mushroom and bryophyte is simply coincidence. On the other hand there are various Galerina species that are always found growing on bryophytes.
Dense bryophyte cushions or mats growing on rotting wood or on soil tend to retain water and so create moist microhabits in the underlying wood or soil – especially in drier areas. Hence there will be fungi, not necessarily bryophilous, that exploit such microhabitats.
You can see an even closer physical association in this photograph, of a few Sphagnum plants from a North European Sphagnum bog, with an obvious white coating present. That coating is the fruiting body of the fungus Tylospora fibrillosa, a species that forms mycorrhizal associations with flowering plants. In the case of Tylospora fibrillosa the underground mycelium commonly produces fruiting bodies on the twig and leaf litter of the forest floor - and occasionally on nearby mosses. The Sphagnum plants in the photo simply provide support for the Tylospora fruiting bodies. Fungi such as Tylospora, markedly two-dimensional in contrast to the obviously three-dimensional mushrooms, are called corticioid fungi (from the Latin word cortex meaning bark or rind) and typically grow on fallen wood. Many corticioid fungi may spread onto bryophytes simply because the bryophytes are growing on the wood within which the fungal mycelium is growing. When the fungal fruiting body starts developing the bryophytes are in the way and so get overgrown. Once again, a coincidental association![]() .
.
A number of mosses grow in colonies that develop into a mounded shape, with much of the mound’s interior composed of trapped, wind-blown debris and dead moss stems and leaves. Such mosses can form quite large clumps on boulders and tree-trunks in well-watered areas, for example the tropics or the wet sclerophyll forests of temperate Australia. This photo ![]() shows a view through a part of a Leptostomum macrocarpum clump. You can clearly see the green, living leaves and (above them) some spore capsules. However, it’s immediately clear that much of the clump is brown - largely composed of dead stems and leaves. You can see some obvious, dead leaves amongst the mass of brown. As the moss stems grow, the lower parts die off. After some time, there would be a significant amount of dead matter within the clump. Various fungi, not necessarily those restricted to bryophytes, feed on such dead matter.
shows a view through a part of a Leptostomum macrocarpum clump. You can clearly see the green, living leaves and (above them) some spore capsules. However, it’s immediately clear that much of the clump is brown - largely composed of dead stems and leaves. You can see some obvious, dead leaves amongst the mass of brown. As the moss stems grow, the lower parts die off. After some time, there would be a significant amount of dead matter within the clump. Various fungi, not necessarily those restricted to bryophytes, feed on such dead matter.
Which fungi?
The bulk of the fungi that most people are familiar with produce fruiting bodies readily visible to the naked eye. Such fungi are colloquially called macrofungi and the mushroom-producing species are undoubtedly the most widely known, but you may well be familiar with puffballs, stinkhorns , coral fungi and cup fungi, to name just a few more types of macrofungal fruiting bodies. There are numerous fungi in which the individual fruiting bodies are hard to see with the naked eye and such fungi are colloquially called microfungi. While useful for descriptive purposes, the terms macrofungi and microfungi are not precisely defined taxonomic terms. The macrofungal fruiting bodies listed above produce sexual spores – via either asci or basidia. Amongst the microfungi there are those which produce sexual spores (mostly within asci, far fewer on basidia) but there are also many which are known to produce only asexual spores. Often you will see the term anamorphic fungi used to label such fungi.
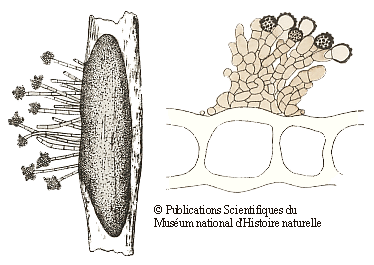
Many of the fungi that grow on bryophytes have quite small fruiting bodies (often under half a millimetre in size) and such fungi need at least a low power microscope to be seen – clearly good examples of microfungi. Many of those bryophilous microfungi are asexual spore producing forms and the following drawings show two examples. The drawings are taken from the publication given in the following reference button and are reproduced by permission of the Muséum national d'Histoire naturelle in Paris. On the left is the fungus Botrytis cinerea. The elongated dark grey object is a sclerotium within a stem of the moss Polytrichum juniperinum. From the sclerotium there are short hyphal outgrowths with clusters of asexual spores at their tips. The moss stem is 1-2 millimetres wide. On the right are brownish hyphae and the knobbly asexual spores of Epicoccum plagiochilae on a leaf of a liverwort in the genus Plagiochila. The drawing shows part of a cross section of the liverwort leaf and you can see several large leaf cells. The leaf is about a twentieth of a millimetre thick![]() .
.
About 350 species of ascomycetes (in over 80 genera) have been found on mosses and liverworts - but what about hornworts. The mycologist Peter Döbbeler has been a major contributor to knowledge about fungi on bryophytes and has noted that hornworts "have not been proven as a substrate for ascomycetes". Amongst the bryophilous ascomycetes perithecia are more common than apothecia. Perithecia provide a more sheltered environment for the spore-producing asci than would apothecia. The gametophytes and sporophytes are generally fairly exposed habitats and so it is not surprising that perithecia are more common. A number of apothecial species have gelatinous apothecia, the gelatinous matrix helping water retention. Another strategy is to grow in the more sheltered areas on bryophyte plants![]() .
.
There are relatively few bryophilous basidiomycetes and most of them are mushroom-producing species. The painting at the top of this page, showing Arrhenia retiruga growing on some moss plants gives you one example. You will also see this species called Leptoglossum retirugum in some printed or internet sources. The gelatinous caps of the mushrooms in genus Arrhenia are no more than a few centimetres in diameter (typically about one centimetre for Arrhenia retiruga ) and the stems are short, or even non-existent in some species with the caps then attached directly to their bryophyte hosts. The gills often have interconnections, giving the underside a maze-like to pored appearance. Some species in the genus grow on wood or soil amongst bryophytes rather than directly on bryophytes. It is thought that the ancestors of at least some of the bryophilous mushrooms were decayers of wood or peaty soil. The possession of wood- or peat-decay enzymes would have facilitated a jump to a bryophyte host![]() .
.
The mushrooms in the genera Gerronema and Rickenella are small, the caps typically no more than a centimetre in diameter (those of Rickenella often only a few millimetres) and the stems mostly less than two centimetres long. The relationships between bryophytes and Gerronema or Rickenella are not always clear but the northern hemisphere species Gerronema marchantiae is one exception, being found exclusively on thalli of thallose liverworts in the genus Marchantia in subpolar and subalpine to alpine areas. A number of Rickenella species are thought to be weakly parasitic on bryophytes. The northern hemisphere Rickenella pseudogrisella is always found growing on the thallose liverwort Blasia pusilla, in arctic or alpine regions. In one study hyphae, identical to those of close-by Rickenella pseudogrisella, could be seen to form clasping pads, or appressoria, on the rhizoids of Blasia pusilla. Additionally, gemmae of Blasia pusilla were found with a sparse covering of fungal spores, identical to the spores to be found on Rickenella pseudogrisella mushrooms, within a few millimetres of the gemmae. Some of those spores had germinated and seemingly infected the gemmae. Also nearby were older, detached gemmae, with short rhizoids covered with fungal appressoria. This evidence strongly suggests both that Rickenella pseudogrisella does "infect" the liverwort and that the fungus can be dispersed with the gemmae. While the physical evidence points to a fungal "infection" of the liverwort that evidence tells us nothing about the nature of the association between the two. Is the fungus purely parasitic or is there some form of partnership with benefits to the liverwort as well? Other Rickenella species are found amongst mosses and you don't need to go to alpine or arctic areas to see the genus. For example, the Eucalyptus Lawn at the Australian National Botanic Gardens in Canberra has lawn, eucalypts and scattered colonies of trailing mosses. In most years you can see at least some Rickenella mushrooms growing there – but only where there are mosses![]() .
.
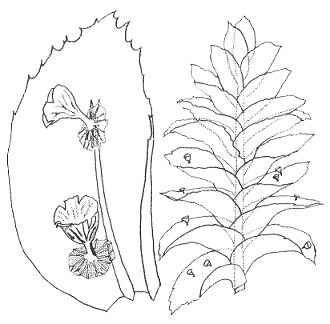
We'll finish this section with even smaller mushrooms. The accompanying drawings of the species Mniopetalum miniatum (below) are taken from the paper given in the next reference button, in which the species was first described. The management of the journal Mycotaxon has kindly allowed the use of the drawings. The mushroom caps are up to about a third of a millimetre in diameter and the stems about a tenth of a millimetre in length and, as in any self-respecting mushroom, there are gills on the underside of the cap. One of the drawings shows a close up of two mushrooms growing on a leaf of the moss Homaliodendron montagneanum and the other shows part of a Homaliodendron plant with mushrooms on several leaves![]() .
.
You've already seen some examples of where on bryophytes you can find bryophilous fungi. The MICRO-NICHES page gives some more examples of the very micro micro-habitats of bryophilous fungi.
Parasitic fungi
A parasitic fungus gets its nutrients from another living organism, with no benefit to the other organism. The bulk of the bryophilous fungi would be parasitic on their hosts since the fungal mycelia are contained within or on the host plants and hence in contact with no alternative food sources. There are varying degrees of parasitism and not all parasitic fungi kill their hosts. In many cases there would be just localized damage. In a similar fashion you are likely to have many parasitic fungi on the plants in your garden that cause only localized damage. As a whole your plants may well be quite healthy but look closely at the leaves. Do you see features such as tiny black spots or small browned off areas? Some such areas may well be the result of localized fungal infections![]() .
.
The mushroom-forming basidiomycete Tephrocybe palustris (formerly known as Lyophyllum palustre) grows on Sphagnum in the northern hemisphere and laboratory experiments have shown that it kills Sphagnum gametophytes. Compared to many of the mushrooms that grow on bryophytes this Tephrocybe is largish – the caps often 2-3 centimetres in diameter and the stem 5 or more centimetres long. The fungus readily attacks and kills healthy Sphagnum plants. The perithecial ascomycete Lizonia baldinii destroys the growing tips and antheridial cups of male plants of mosses in the family Polytrichaceae and the host plants die from the tip down![]() .
.
The fungus Eocronartium muscicola is another northern hemisphere parasitic basidiomycete and has been found on about twenty moss species. The fruiting bodies are not mushrooms but simple, white stalks up to a centimetre or so in length and under a millimetre in diameter. In those cases where the moss-Eocronartium association has been carefully examined the fungus generally infects mosses only where embryonic sporophytes have started developing and the fungus displaces the developing sporophytes. The fungus appears to initially develop around the foot of the sporophyte and so gets access to the nutrients passing from the moss gametophyte to the sporophyte. The fungus doesn't kill gametophytes.There's more information in the EOCRONARTIUM MUSCICOLA CASE STUDY.
There are various fungi which grow directly on bryophytes but which have never been found to penetrate the bryophyte cells nor cause any obvious damage by any other means. It may be that such fungi use nutrients that leach out of the host bryophytes. For example, experimental studies have shown that many dry, dormant bryophytes leak nutrients in the initial stages of re-hydration. You've seen that the fungi that grow on bryophytes typically produce small fruiting bodies. A mycelium confined to bryophyte gametophytes or sporophytes has a fairly limited food supply, which means that limited resources are available for the production of fruiting bodies.
Nearby but connected
Early on this page you saw that just because a fungal fruiting body appears on a bryophyte doesn't automatically mean that the fungus is using the bryophyte as a food source. Conversely, the fact that a fungal fruiting body is not in obvious contact with a bryophyte doesn't mean that there is no hidden connection.
Ascomycetes of the genera Lamprospora, Neottiellia, Octospora and Octosporella produce disk-like apothecia as fruiting bodies. They are often brightly coloured in shades of orange or reddish orange and are mostly just a few millimetres in diameter, though up to a centimetre in some cases. Species in these genera parasitize bryophytes. While the apothecia may sometimes appear on bryophytes, they are mostly found on soil near bryophytes. In the latter case the parasitism is not obvious. In fact the parasitism is via the bryophyte rhizoids. Hence the fungus-bryophyte connection is out of sight, in the soil. In order to confirm the association, it has been necessary to carefully trace the mycelial connection between the fruiting bodies and the rhizoids and observe the hyphal penetration of the latter. It is important to realize that there are other, non-parasitic, fungal genera which produce apothecia amongst bryophytes on soil, and where the apothecia are of similar colours and sizes as those reported above. Finally, while the genera listed at the beginning of this paragraph are mostly parasitic via rhizoids, some will also parasitize the persistent protonemata of some mosses and Octospora gemmicola infects the tubers (or rhizoidal gemmae) of Bryum atrovirens![]() .
.
Bryophytes exploiting fungi
While there are many parasitic fungi that exploit bryophytes, there are also bryophytes that exploit fungi.
The simple thalloid liverwort Cryptothallus mirabilis lacks chlorophyll, has a white thallus (which has led to it sometimes being called a “ghostwort”) and grows beneath the litter layer on the forest floor. Given its buried habitat, this liverwort is easily overlooked and so its full geographic range is probably unknown. Thus far it has been found in Europe, Greenland and Costa Rica. It relies on one or more associated fungi for its food. Studies into the fungal partners have shown that one or more species in the corticioid genus Tulasnella are involved. These fungi form mycorrhizal associations with various photosynthesizing plants and Cryptothallus “steals” carbon from the fungi but gives nothing in return. The photosynthesizing plants are the primary food source and, in essence, a Cryptothallus plant is indirectly parasitic on some photosynthesizing plant - with the fungus simply an intermediary![]() .
.
The species in the genus Haplomitrium are thought to constitute a primitive group of liverworts - a relict of an ancient line of liverworts and related only remotely to other present-day species. Haplomitrium gametophytes develop short, root-like growths in which there is a fungus/liverwort association. These root-like growths are called mycotrophic axes (that last word being the plural of axis) and are quite distinct from rhizoids (the root-like anchoring structures produced by many bryophytes). Species of Haplomitrium lack rhizoids. Each mycotrophic axis secretes abundant mucilage, which harbours fungal hyphae. The hyphae penetrate the axis and, depending on the liverwort species, may form swellings or small branched growths (the latter called arbuscles) within the cells of the axis.
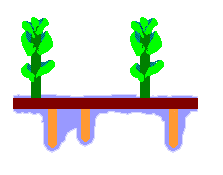 The accompanying diagram is a stylized depiction of the gametophyte of Haplomitrium gibbsiae. In Australia the species is found in Tasmania. The colours have no significance, other than to help distinguish the features. The upright, photosynthesizing shoots are shown in green. These have grown up from a creeping rhizome (shown in dark brown), which has also produced the downward-growing mycotrophic axes (shown in orange-brown). Surrounding the mycotrophic axes is the mucilage (shown in blue).
The accompanying diagram is a stylized depiction of the gametophyte of Haplomitrium gibbsiae. In Australia the species is found in Tasmania. The colours have no significance, other than to help distinguish the features. The upright, photosynthesizing shoots are shown in green. These have grown up from a creeping rhizome (shown in dark brown), which has also produced the downward-growing mycotrophic axes (shown in orange-brown). Surrounding the mycotrophic axes is the mucilage (shown in blue).
There is some variation in the gametophytic structure between different Haplomitrium species. In Haplomitrium gibbsiae mycotrophic axes are rarely more than a few millimetres long and do not form side-branches. In Haplomitrium ovalifolium (which has not been found in Australia) there is no rhizome, the mycotrophic axes growing directly from the bases of the upright shoots. In the latter species the mycotrophic axes are thinner, longer and with numerous side-branches.
The available, but indirect, evidence certainly suggests that the fungus obtains carbohydrates from the liverwort and that the liverwort obtains other nutrients from the fungus. However, confirmation of that suggestion would need a detailed investigation of the physiology of the fungus-liverwort association.
The fungi involved in the association belong to a group called the Glomeromycota, considered to be the most ancient of the mycorrhizal fungi. Given that, and the fact that Haplomitrium is an ancient plant lineage, it has been suggested that the Haplomitrium-Glomeromycota association is the most primitive of the known symbioses between fungi and land plants. The Glomeromycota form associations with a number of thallose liverworts![]() .
.
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)